Abstract
Introduction
Chimeric antigen receptor T cell (CAR-T) expansion has been consistently identified as a predictor of clinical response. However, there are no clinically available test to measure CAR-T presence after infusion for the FDA approved CAR-T therapy. We hypothesize that the lymphocyte expansion which can be readily measured as absolute lymphocyte count (ALC) in blood count differential could be a clinically accessible surrogate for CAR-T expansion in patients who receive FDA approved CD19 CAR-T to treat aggressive B-cell non-Hodgkin lymphoma (NHL). We examined the ALC levels in the first 2 weeks after CAR-T infusion and correlation with clinical outcomes in NHL patients who received CAR-T at our institution.
Methods
Records were reviewed for patients who received CAR-T between 6/2016 and 1/2021 at Mayo Clinic, Rochester. CAR+ T cells were identified by flow cytometry using an anti-FMC63 antibody. Receiver operator curve was generated using nominal logistic regression to predict complete response (CR) as best response. Progression-free survival and overall survival were calculated using Kaplan-Meier method and between-group differences were assessed using log-rank test. Continuous variables were compared using wilcoxon text and categorical variables were compared using chi-square test.
Results
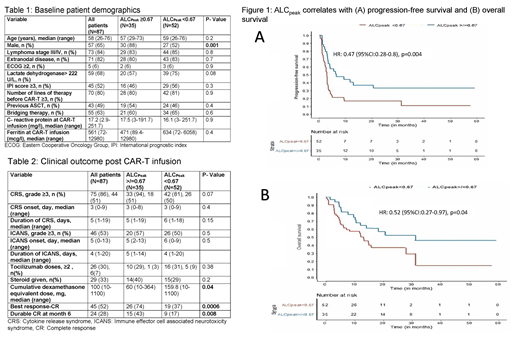
Among the 87 patients who received CAR-T for NHL, majority of the pts received axicabtagene ciloleucel (86, 99%). The highest ALC level (ALC peak) in the first 15 days were identified for all patients and median ALC peak was 0.44 X10 9/L (range, 0 - 2.55x10 9/L). The median time to ALC peak was 10 ±3 days. Increasing ALC peak levels correlated with increased CAR+ T cells in blood measured by flow cytometry (n=16, R=0.63, p=0.0008). Using ROC analysis, an ALC peak level of 0.67 was identified as the cut point for best association with CR (AUC=0.68, p=0.0004). Baseline demographics were similar between the high ALC peak (N=35) and low ALC peak (N=52) groups, as shown in Table 1. There was no difference in the incidence, high grade, or duration of cytokine release syndrome (CRS) and immune effector associated neurotoxicity syndrome (ICANS) between the two groups (Table 2). Patients who received a higher cumulative dose of steroid for management of CRS and ICANS had lower ALC peak. A higher ALC peak was associated with an increased CR rate and durable CR at month 6. Similarly, a higher ALC peak was associated with increased progression-free survival (PFS, 6.4 months vs. 2.7 months, p=0.004) and overall survival (OS, 31 months vs. 12 months, p=0.04), as shown in figure 1.
Conclusion
Given the typical CAR-T expansion seen in the first 2 weeks post infusion, ALC peak in the first 15 days is a clinically accessible, reliable surrogate for CAR-T expansion and predicts durable CR and longer PFS, OS.
This study was supported in part by the Mayo Clinic Center for Individualized Medicine, Bernard E. and Edith B. Waterman, Henry J. Predolin Foundation and other generous benefactors of Mayo Clinic.
Bennani: Verastem: Other: Advisory Board; Purdue Pharma: Other: Advisory Board; Daichii Sankyo Inc: Other: Advisory Board; Kyowa Kirin: Other: Advisory Board; Vividion: Other: Advisory Board; Kymera: Other: Advisory Board. Paludo: Karyopharm: Research Funding. Wang: Genentech: Research Funding; Eli Lilly: Membership on an entity's Board of Directors or advisory committees; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; MorphoSys: Research Funding; InnoCare: Research Funding; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees. Ansell: Bristol Myers Squibb, ADC Therapeutics, Seattle Genetics, Regeneron, Affimed, AI Therapeutics, Pfizer, Trillium and Takeda: Research Funding. Lin: Janssen: Consultancy, Research Funding; Legend: Consultancy; Bluebird Bio: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Vineti: Consultancy; Gamida Cell: Consultancy; Kite, a Gilead Company: Consultancy, Research Funding; Novartis: Consultancy; Takeda: Research Funding; Merck: Research Funding; Sorrento: Consultancy; Juno: Consultancy.